Quality Department
Quality is at the heart of our business, it’s essential in every action, every gesture in the office and in the warehouse.
Everything is traced, recorded, tracked and archived.
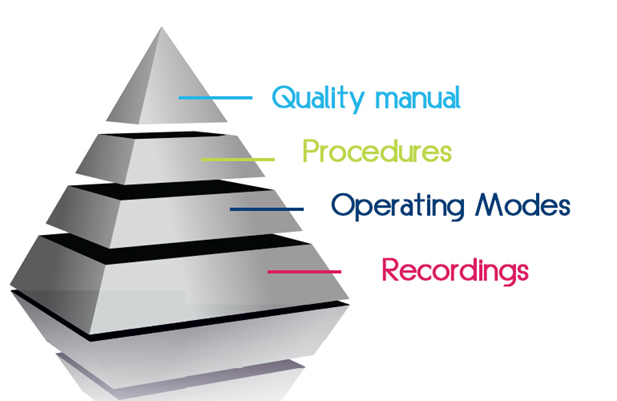
Architecture of our quality approach
- The quality manual consists of procedures, operating modes and recording sheets.
- Procedures are organizational records that meet the regulations.
- Operating Modes describe precisely how to perform a task
- Records are evidence of the operations we have conducted
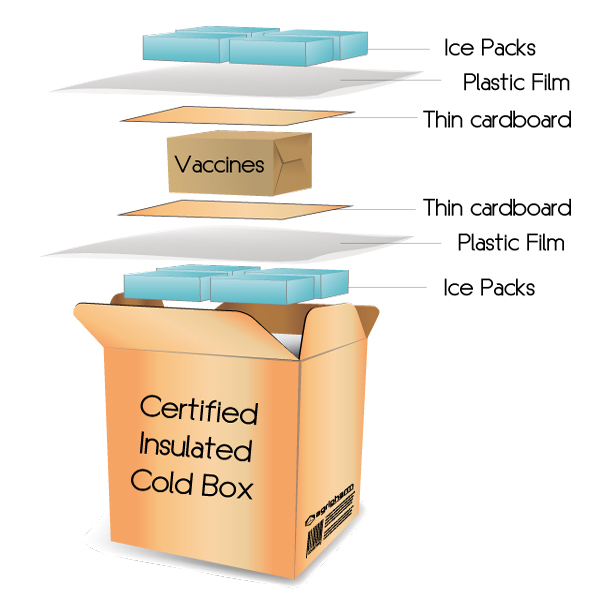
A defined and validated procedure
The cold packaging is established according to a procedure approved by our quality service. Our cold boxes are closed in our cold rooms, just before their departure, and the vaccines are guaranteed to stay in the cold chain.
- Control of storage temperatures during transport upon receipt of vaccines
- Regular monitoring of cold shipments by special tracers
- Continuous monitoring of cold rooms by LABGUARD system
- Alarm system for the deviating temperatures in the cold rooms
The number of ice packs is evaluated depending on the type and the volume of the cold box and depending on the season (Winter and Summer)
Special tracers for regular monitoring of cold boxes and active cooled transport.